
Carbon has an electronegativity of 2.5, while the value for hydrogen is 2.1. Describe the electronegativity difference between each pair of atoms and the resulting polarity (or bond type). A bond is said to be a polar bond,if it has. Electronegativity values tend to increase as you go to the right and up on the table shown in the image below. Electronegativity is a qualitative measure of how much an atom attracts electrons in a covalent bond. Polarity in organic chemistry refers to a separation of charge and can describe a bond or an entire molecule. To help determine the electronegativity of an atom, look at the periodic table of elements. measure of how equally or unequally electrons are shared in a covalent bond equally shared electrons have bonds that are. Bond polarity is determined by the difference in electronegativity and is defined as the relative ability of an atom to attract electrons when present in a compound. The unequal charge distribution results in the net dipole moment. Electronegativity is the power of an atom in a molecule to attract electrons to itself. The polarity of a molecule is decided based on the unequal charge distribution of the atoms involved in the molecule. They become reality soluble in water because of successfully competing with the help of Hydrogen bonds between the water molecules. Polar molecules tend to attract water molecules, particularly through a Hydrogen bond. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of. Polarity of a Water Molecule Water ( H 2 O) is polar because of the bent shape of the molecule.
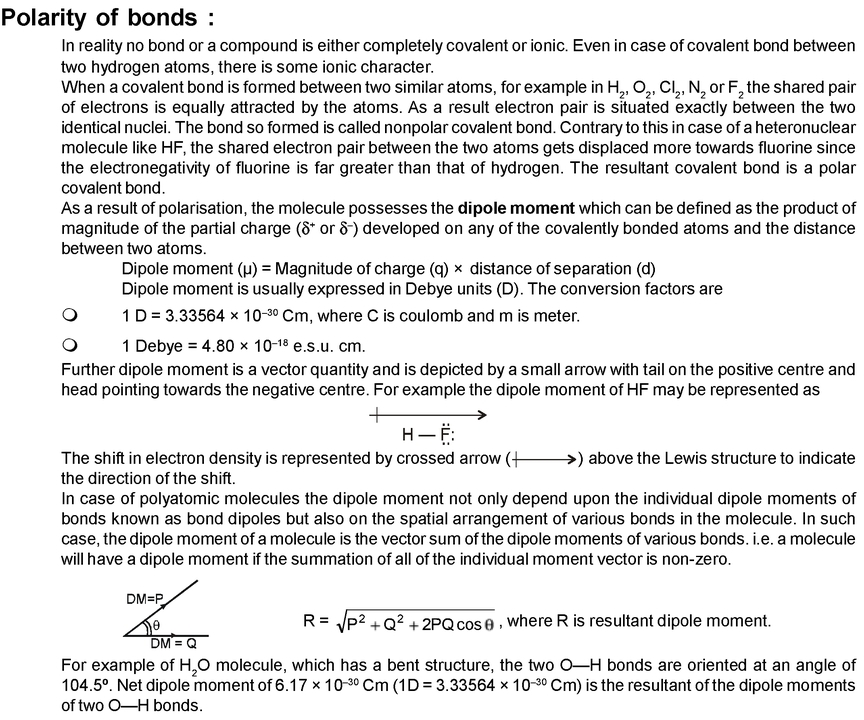
To help determine whether a bond is polar or nonpolar, you must know the electronegativity. The polarity of a molecule majorly depends on its constituent atoms and their arrangement around the central atom. These electrons repel each other, bending the O-H bond away from the linear angle. But if an electron is more attracted to one atom than another, the bond is polar. If the electron is shared equally between the atoms in a covalent bond, then the bond is nonpolar. Covalent bonds are formed between one or more pairs of shared electrons. Ionic bonds are formed between two oppositely charged ions, such as a metal and a nonmetal. There are two basic types of bonds, covalent and ionic. To know the structure, you must first know how the atoms and molecules are arranged. The original Pauling formulation of electronegativity cannot account for all possible bonding schemes.Why are some gases greenhouse gases and others are not? The structure of a molecule influences the way it interacts with infrared radiation. I am not an expert on the development and application of electronegativity scales, but suffice it to say that these scales are (1) empirical and (2) provide useful guidelines.

Here the carbon has a very slight negative charge relative to oxygen, as predicted based on the computation of formal charges. AQA pre-2016 papers Bond Polarity 1 MS Bond Polarity 1 QP Bond Polarity 2 MS Bond Polarity 2 QP Bonding & Physical Properties 1 MS Bonding & Physical. The OP presented an excellent counterexample: CO. On the other hand inductive effects should not be dismissed. In any case, when considering bond polarity, electronegativity overrules formal charge. This unequal distribution of electrons is known as a polar covalent bond, characterized by a partial positive charge on one atom and a partial negative charge. It is similar to the difference between formal charge and oxidation state, as well explained in the Wikipedia.Ī better picture (closer to the real electronic distribution) is, as Pauling would probably suggest, intermediate to the two, assuming a hybrid between possible Lewis structures (covalent and ionic). It provides examples so you can quickly distinguish nonpolar molecules from. This does not account for differences in electronegativity. 28K Share 2M views 7 years ago This video provides a fast way for you to determine if a molecule is polar or nonpolar. When you compute formal charges you split bonding electrons evenly between bonded atoms.


 0 kommentar(er)
0 kommentar(er)
